In Aqueous Solution Classify These Compounds as Strong
Strong Acid- HNO_3. I was think A was strong B and C were weak and D is non.
Solved In Aqueous Solution Classify These Compounds As Chegg Com
In aqueous solution classify these compounds as strong acids weak acids strong bases or weak bases.

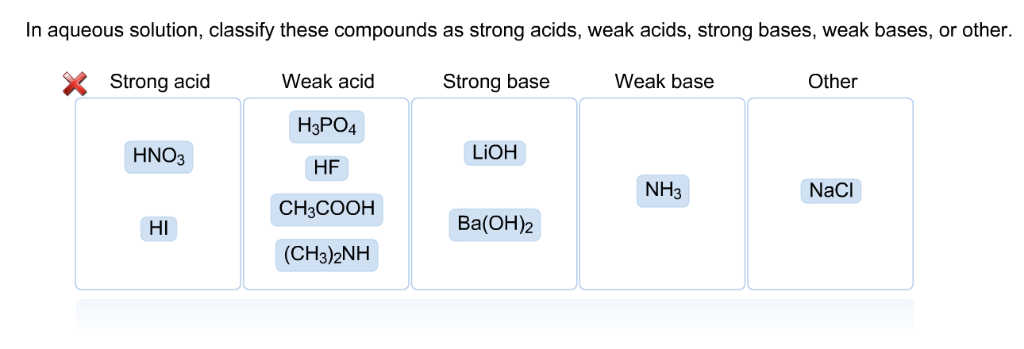
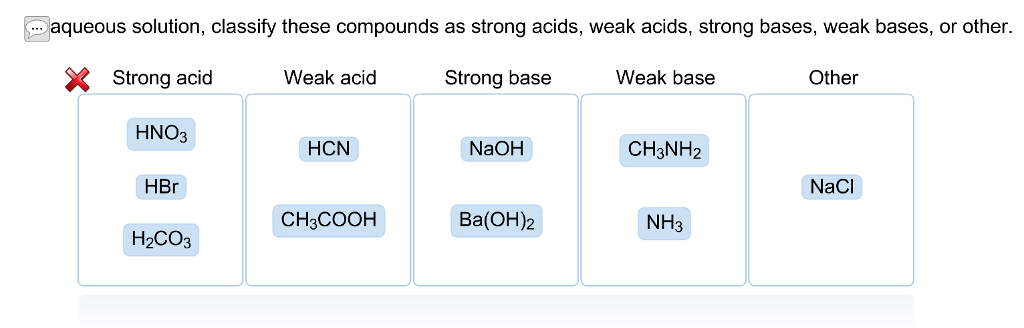
. Even insoluble ionic compounds eg AgCl PbSO 4 CaCO 3 are strong electrolytes because the small amounts that do dissolve in water do so principally. In aqueous solution any base stronger than OH is leveled to the strength of OH because OH is the strongest base that can exist in equilibrium with. Strong acid Weak acid Strong base Weak base Other ΗΝΟ CHNH HPO HBr КОН CHCOOH HCN Вa ОН NH NaCI Answer Bank.
CH_3COOH Strong Base- NaOH. In aqueous solution classify these bartleby. In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other.
In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other. NH_3 Other- NaCl bc it is a salt Assuming equal concentrations rank these solutions by pH. Keep in mind that you will be encountering three types of compounds and aqueous solutions.
Strong acid Weak acid Strong base Weak base HNO HBr NaOH NII I1co HF. In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other. In aqueous solutions H_3O is the strongest acid and OH is the strongest base that can exist in equilibrium with H_2O.
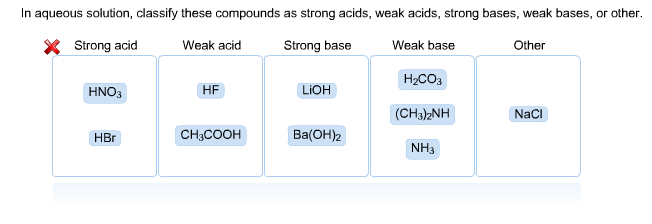
Read Free Strongest Acid In Aqueous Solution OH is the strongest base that can exist in equilibrium with H_2O. In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other. Other HCN NaOH BaOH 2 CH 3 3 N NH 3 CH 3 COOH NaCl HNO 3 H 3 PO 4 HBr.
In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other. NaNO3s Naaq NO3aq Molecular Compounds. In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other.
In an aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other. These are usually non-electrolytes. Classify each of the following compounds as a strong acid weak acid strong base or weak base and write the Ka expression for any weak acid.
In aqueous solution classify these compounds as strong acids weak acids strong bases or weak bases. CH3COOH H3PO4 HF Strong Base. In aqueous solution any base stronger than OH is.
HI Weak Acid-H_2CO_3. The leveling effect applies to solutions of strong bases as well. MXs M aq X aq States of matter are expressed as s.
AH2SO4 bCuS cHF dZnNO32 Which are strong weak or non electrolytes. You have not correctly placed all the acids. HNO3 HBr HF CH3COOH H3PO4 NaOH.
They can be expected to dissociate 100 in aqueous solution. Strong electrolytes are usually ionic compounds. In an aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other.
Weak or non-electrolyte when dissolved in water aqueous solution. HI HNO3 Weak Acid. Strong electrolytes dissociate completely when dissolved in solution and conduct electricity very well.
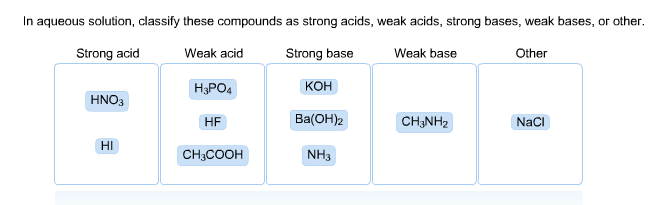
Strong acid Weak acid Strong base KOH BaOH2 Weak base Other H3PO4 HNO3 HF CH3NH2 NaCI HI CHjCOOH NHt. HNO3 HBr HF CH3COOH H3PO4 NaOH BaOH2 CH33N NH3 NaCl. These are usually strong electrolytes and can be expected to 100 dissociate in aqueous solution.
Electrolytes are compounds that dissociate into their component ions and conduct electricity in aqueous solution. NaOH BaOH2 Weak Base. In an aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other.
They do not dissociate to form ions. The leveling effect applies to solutions of strong bases as well. Terms in this set 18 In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other.
Electrolytes are classified by the extent to which they dissociate and how well they conduct electricity. Strong acidWeak acid Strong base HCN LiOH NH3 Weak base Other HNO3 H3PO4 BaOH2 NaCI HBr CH3COOH CH32NH. In aqueous solutions H_3O is the strongest acid and OH is the strongest base that can exist in equilibrium with H_2O.
In an aqueous solution classify these bartleby. The leveling effect applies to solutions of strong bases as well. In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or The following solution is suggested to handle the subject In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases orLets keep an eye on the content below.
Strong acid Weak acid Strong base Weak base Other Answer Bank NH NaCl HNO3 Ba OH2 CH22N CHCOOH HCO3 KOH HBr HCN. Strong acid Weak acid Strong base Weak base Answer Bank Вa ОН HBr NH HNO HF NaOH HCO. Strong acid Weak acid Strong base Weak base HNO3 KOH NH4Cl CH3NH2 HBr Ba OH2 H3PO4 NaF HF Incorrect.
Acid formulas tend to start with H The best way to distinguish strong. We can classify compounds as strong weak or nonelectrolytes by measuring the conductivity of these chemical solutions. In aqueous solution classify these compounds as strong acids weak acids strong bases weak bases or other.
BaOH_2 Weak Base- CH_3NH_2. Classify these compounds by whether they behave as strong acids weak acids strong bases or weak bases in aqueous solution. All ionic compounds are strong electrolytes because they mostly break up into ions as they dissolve in water.
Nonelectrolytes do not ionize at all. In aqueous solution any base stronger than OH is leveled to the strength of OH because OH is the strongest base that Page 411.
Solved In Aqueous Solution Classify These Compounds As Chegg Com
Solved In Aqueous Solution Classify These Compounds As Chegg Com
Solved An Aqueous Solution Classify These Compounds As Chegg Com
No comments for "In Aqueous Solution Classify These Compounds as Strong"
Post a Comment